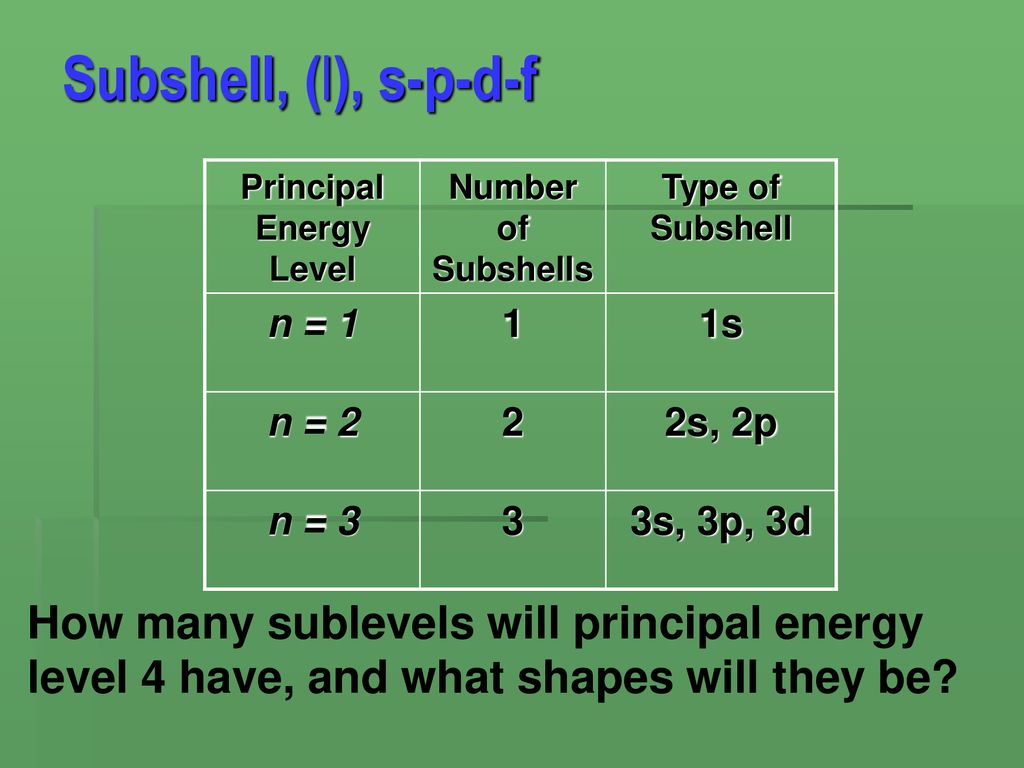
L Energy Level Sublevels
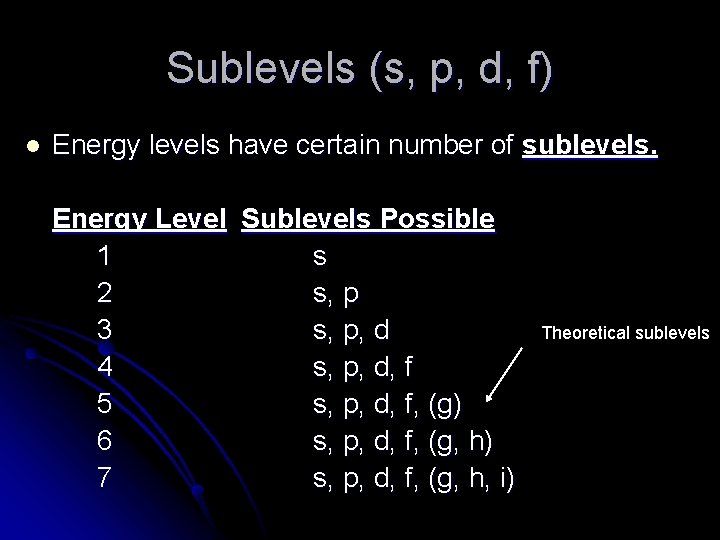
Energy levels in an atom can be numbered 1 2 3. Level 4 has 4 sublevels - s p d and f.
Energy Levels Sublevels Electrons
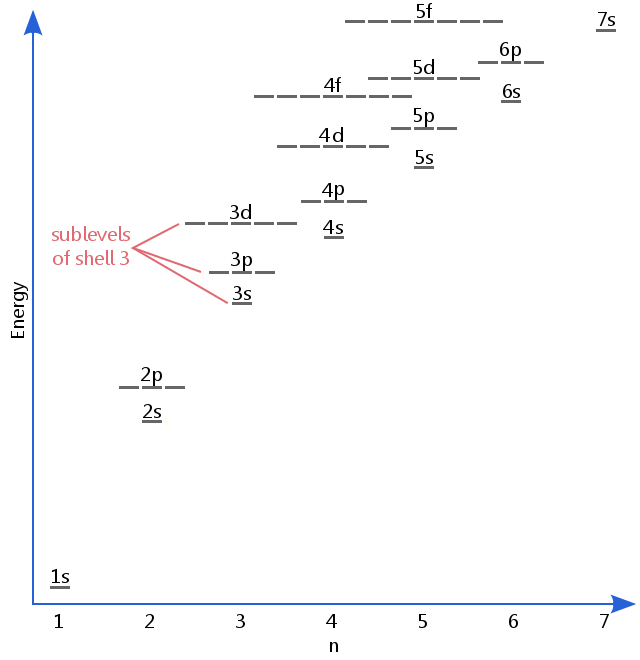
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d.
L energy level sublevels. Orbitals Atomic Energy Levels Sublevels Explained - Basic Introduction to Quantum Numbers - YouTube. The number of orbitals in a subshell is therefore 2l 1. Electron Energy Level 1 In each energy level electrons fill sublevels in a certain order Level 1.
Niels Bohrs earliest quantum theory said that electrons occupy spherical shells centered on the atomic nucleus such as the two electron shells. Therefore the second level can contain a maximum of eight electrons that is two in the s. Level 2 has 2 sublevels s and p.
1 is the lowest energy level closest to the nucleus and energy level infinity corresponds to the energy of an electron with is not attracted to the nucleus at all. How do you arrange sublevels in order of increasing energy. Similarly how many sublevels are in the 5th energy level.
It should be three sublevels the s p and d sublevels. Figure 965 shows the order of increasing energy of the sublevels. What is the maximum number of sublevels in the third principle energy level.
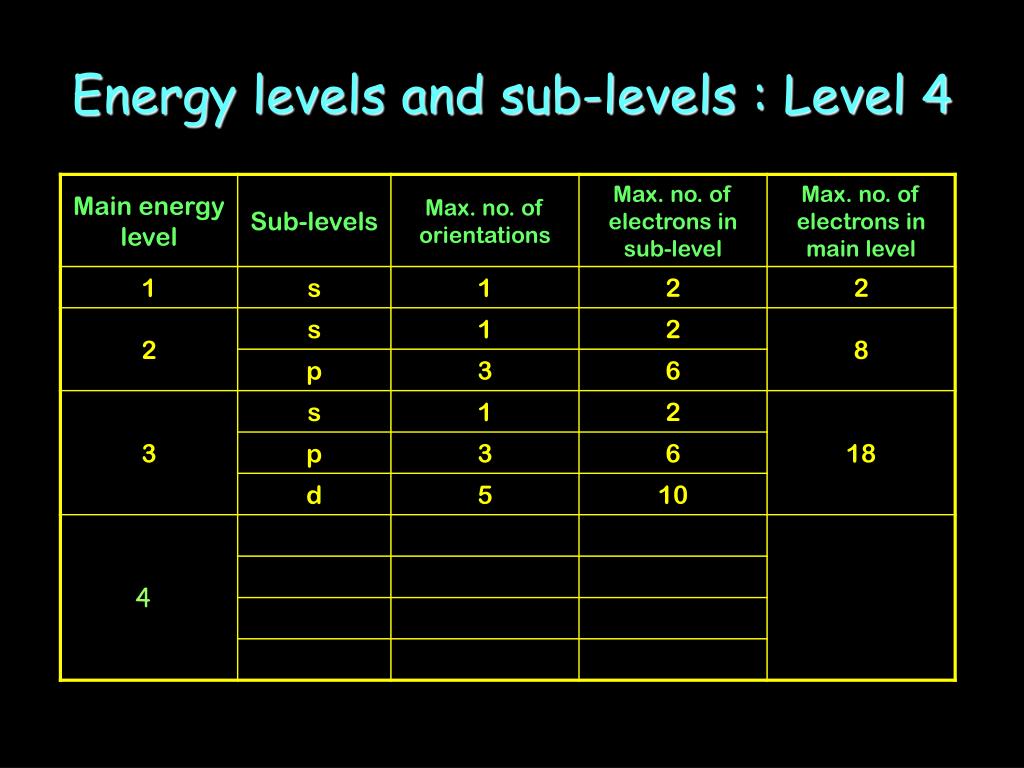
A set of three p orbitals called the p sublevel can hold a maximum of six electrons. Level 3 has 3 sublevels - s p and d. In chemistry sublevels refer to energies associated with electrons.
The second energy level has s and p sublevels. Each energy level has certain sublevels. Near the nucleus the energy level is least and goes on increasing on furthering from nucleus.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Which has higher energy 6s or 4f. The chart below shows the sublevels that make up the first four energy levels.
The lowest energy sublevel is always the 1s sublevel which consists of one orbital. Each principal energy level above the first contains one s orbital and three p orbitals. 1s 2s 2p 3s 3p now next is 3d BUT the d orbitals are complex and rather high.
The single electron of the hydrogen atom will occupy the 1s orbital when the atom is in its ground state. Number of energy levels in each period The atoms in the first period have electrons in 1 energy level. The atoms in the second period have electrons in 2 energy levels.
You need to memorize these 4- sublevels. Orbitals Atomic Energy Levels Sublevels Explained - Basic Introduction to Quantum. What is the maximum number of sublevels in the third principle energy level.
Orbital shapes are defined as the surface that contains 90 of the total electron probability. The energy levels thus converge as they approach infinity. In physics sublevels may also refer to energies associated with the nucleus.
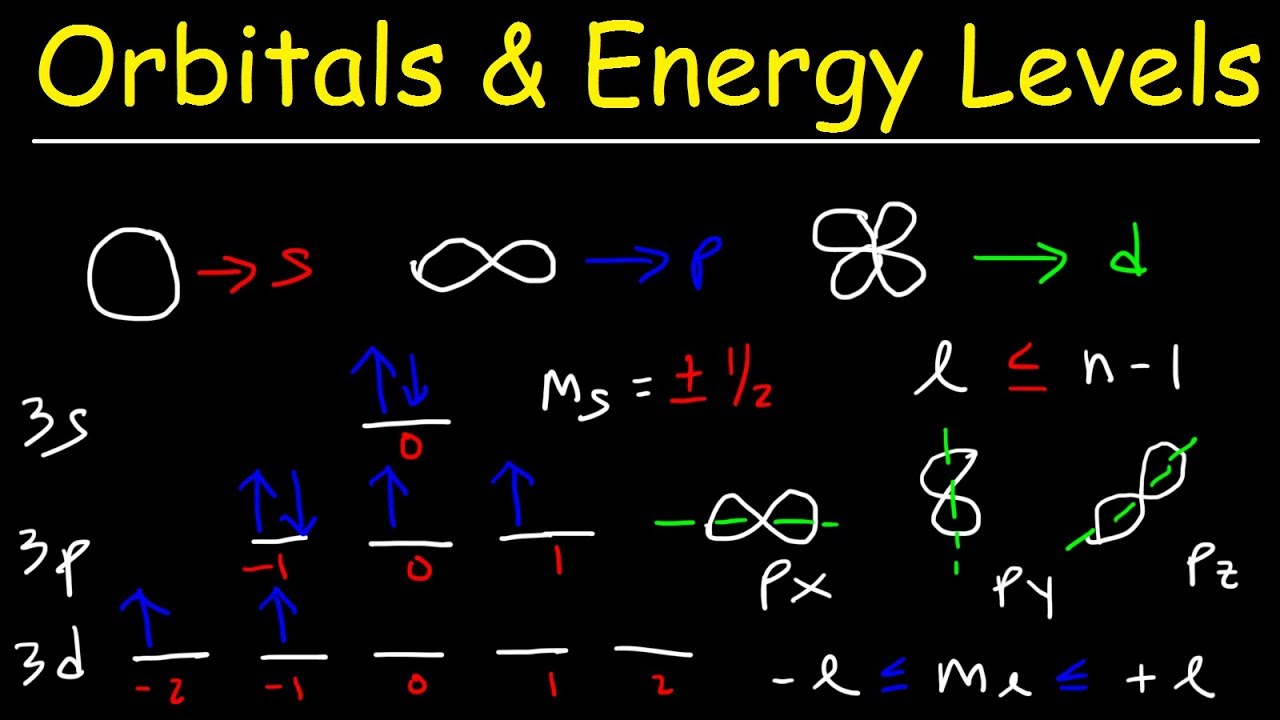
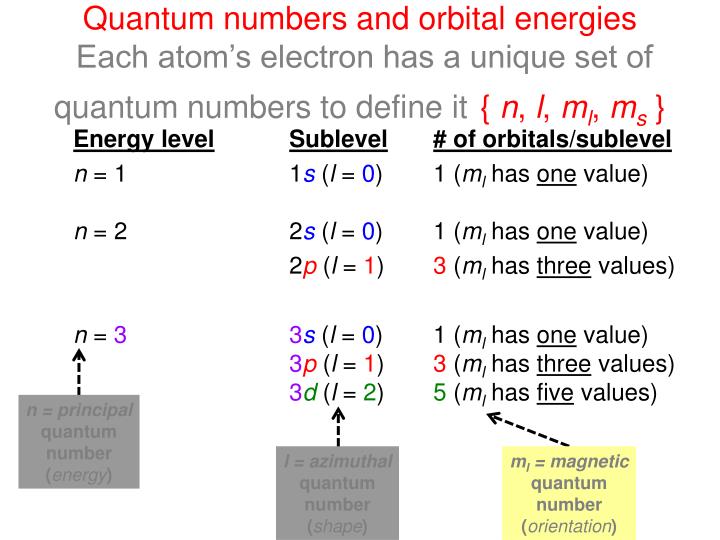
The sublevels are represented by the letters s p d and f. A sublevel is an energy level defined by quantum theory. Energy level Sublevel of orbitalssublevel 1 Quantum numbers and orbital energies Each atoms electron has a unique set of quantum numbers to define it n l ml ms.
Each energy level or shell is divided into sublevels. Level 2 has 2 sublevels - s and p. The atoms in the third period have electrons in 3 energy levels.
The sublevels are labeled s p d and. The order of increasing energy of the sublevels. The atoms in the fourth period have electrons in 4 energy levels.
The terms sublevel and subshell are used interchangeably. Order of energy is given by nl. N 1 1s l 0 1 ml has one value n 2 2s l 0 1 ml has one value 2p l.
The angular momentum quantum number generally symbolized by l denotes the orbital. What is the total number of sublevels in the second principal energy level. To see more answers head over to College Study Guides.
There is one orbital in an s subshell l 0 three orbitals in a p subshell l 1 and five orbitals in a d subshell l 2. How many sublevels are in energy level. Level one has one sublevel an s.
This order corresponds to the order in which the energy sublevels are filled by electrons. The first energy level has an s sublevel. Energy level Sublevel of orbitalssublevel.
Only has one s sublevel a spherical shape. Level one has one sublevel an s. Level 3 has 3 sublevels s p and d.
We fill in electrons according to lowest energy sublevels first. So we basically go in order.
Ppt Energy Levels And Sub Levels Powerpoint Presentation Free Download Id 2061109
Definition Of Sublevel Chemistry Dictionary
What Are The Orbitals Present In The Fifth Principal Energy Level
Orbitals Atomic Energy Levels Sublevels Explained Basic Introduction To Quantum Numbers Youtube

A Energy Level Diagram Showing The Hyperfine Sublevels Of The Download Scientific Diagram

Electron Configurations Quantum Theory Electrons Are Found In Orbitals Defined By Quantum Numbers N L And M Like Seats In A Theatre Organized In Section Ppt Download
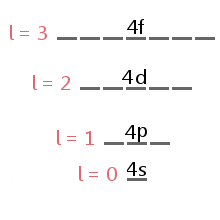
Definition Of Sublevel Chemistry Dictionary
Ppt Quantum Numbers And Orbital Energies Each Atom S Electron Has A Unique Set Of Quantum Numbers To Define It N L Powerpoint Presentation Id 292706
The Nature Of Light Honors L Dual Nature
Energy Levels Sublevels Electrons

Electrons In Atoms Ppt Download
Posting Komentar untuk "L Energy Level Sublevels"