Energy Level
Increasing your energy levels will often have as much of an impact on your cognition as the most powerful nootropics. In chemistry energy is a measure of how stable a substance is.

Total 2 Average 3 X2f 5 How Does The Energy Level Diagram Show This Reaction Is Exothermic Energy P Chemical Energy Exothermic Reaction Energy Level
Notice that I set E to be a positive quantity and the systems energy is E.

Energy level. The equations are thus rewritten as. In this article well look at how you can boost energy levels in a safe and sustainable way using natural supplements. Because each excited rovibrational level is coupled to a number of lower lying rovibrational levels in other electronic states by spontaneous decay or other relaxation mechanisms the system is unavoidably.
Total energy of an electron in Bohrs nth stationary orbit is. The energy levels created in the energy gap by doping elements other impurities or defects are due to the disruption of the perfect crystal structure and those atoms or defects can usually capture electrons or holes. The change of a level from being electron-unoccupied to being electron-occupied corresponds to making the impurity atom or defect more negative by one electron charge.
The closest shell to the nucleus is called the K shell followed by the L shell then the M shell and so on away from the nucleus. E n - frac2πmK2Z2e4n2h25 Or E n - frac136n26 Here TE of an e-in a stationary orbit is negative which means the electron is tightly bound to the nucleus. The highest energy level that an electron can occupy at the absolute zero temperature is known as the Fermi Level.
Energy level definition is - one of the stable states of constant energy that may be assumed by a physical system used especially of the quantum states of electrons in atoms and of nucleicalled also energy state. Giving orbital electrons energy. In the second case the kinetic energy is greater than zero for x a and negative otherwise since the total energy is negative.
This is a huge mistake. The Fermi level lies between the valence band and conduction band because at absolute zero temperature the electrons are all in the lowest energy state. The lower the energy level of an electron the more stable the electron is.
Each molecular electronic state consists of a multitude of rovibrational levels. Energy has an enormous effect on your physical and mental performance. In chemistry an electron shell or energy level may be imagined as an orbit with electrons around the nucleus of an atom.
Thus an electron would be in its most stable state when it. Compared to atomic systems the molecular energy level structure and relaxation mechanisms are much more complex. We also assume that E.
1 2 d ψx 2 2 d. Once an electron has gained enough energy to reach the E 0 level it can leave the atom leaving it ionised. The shells can be denoted by alphabets K L M or quantum numbers n 1 2 3 4 and.
The energy levels are shown as negative because we are imagining the electrons in a well due to the attraction of the protons in the nucleus from which it has to gain energy to climb.

Principal Energy Level Easy Science Energy Level Easy Science Science Student

Pin By Noah Melon On Games What Is Energy Energy Level Energy Activities

Electron Energy Levels Electron Configuration Chemistry Teaching Science

Pin On Sat Ii Chemistry Test Questions

Levels Of Energy Consciousness Google Search Energy Consciousness Consciousness Energy Level

Energy Level Easy Science Energy Level Same Energy Energy

Bohr Model Of Atom Energy Level Diagram For Hydrogen Atom Energy Level Bohr Model Diagram

Atomic Structure Shells Klmn With Energy Levels With Respect To The Formula Of 2nsquare Energy Level Chemistry Atomic Structure

Energy Levels As The Day Progresses What S So Funny Energy Level Funny Quotes

Sublevel Easy Science Electron Configuration Energy Level Aufbau Principle
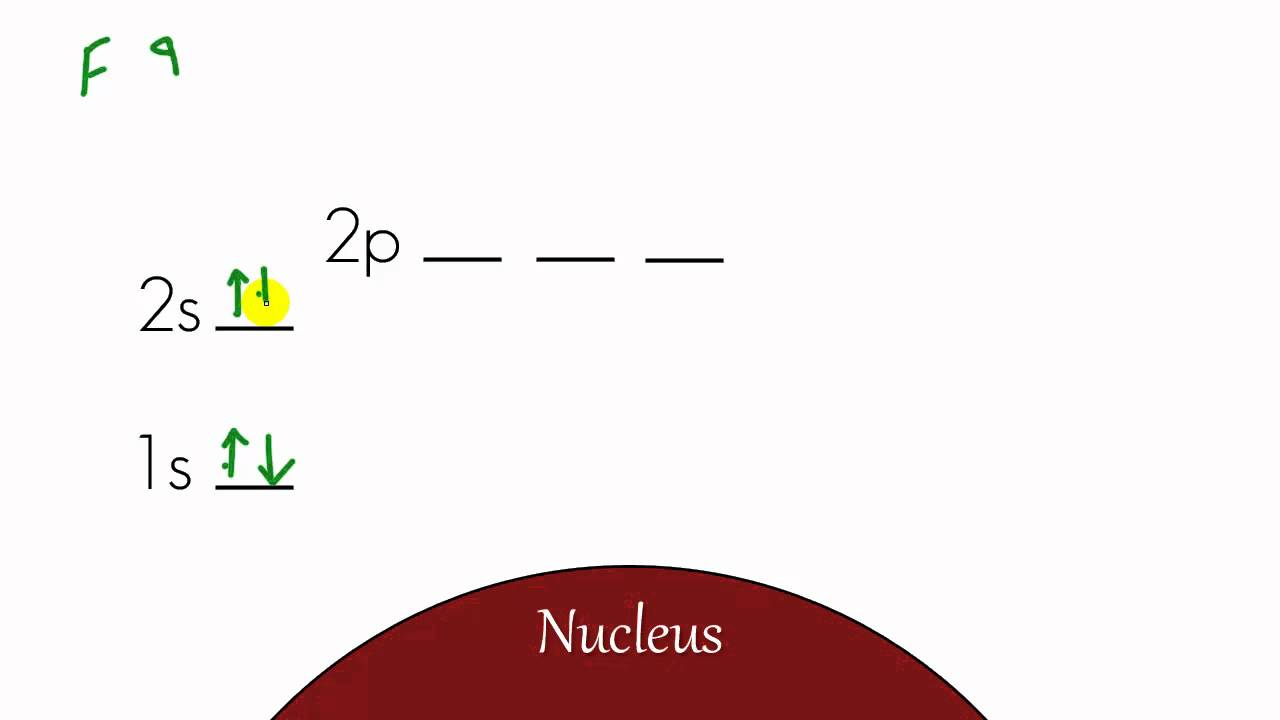
Chemistry Lesson 12 Energy Level Diagram And Electron Configuration Youtube Chemistry Lessons Electron Configuration Chemistry
Posting Komentar untuk "Energy Level"